Reaction 1:
CuO(s)→Cu(s)+12O2(g)ΔG°=155kJ/molrx n
A chemist wants to produce Cu(s) from...

Chemistry, 17.06.2021 01:00, jazzy200076
Reaction 1:
CuO(s)→Cu(s)+12O2(g)ΔG°=155kJ/molrx n
A chemist wants to produce Cu(s) from a sample of pure CuO(s) according to reaction 1, represented by the equation above.
(a) Using the data in the following table, calculate the value of the standard entropy change, ΔS°, for the reaction.
Substance Absolute Entropy at298 K (JK−1mol−1)
Cu(s) 33
O2(g) 205
CuO(s) 43
(b) Given that ΔG° for reaction 1 is positive (155kJ/molrxn), what must be true about the sign of ΔH° for the reaction? Justify your answer.
The decomposition of CuO(s) into Cu(s) and O2(g) is not a thermodynamically favored reaction. However, to produce Cu(s) from CuO(s), the chemist decides to pass H2(g) gas over the CuO(s) as it is heated strongly, as represented below.
Reaction 2:
CuO(s)+H2(g)−-heat→ Cu(s)+H2O(g)
(c) Reaction 2 is what type of chemical reaction? Justify your answer.
A third chemical reaction is represented below.
Reaction 3:
H2(g)+12O2(g)→H2O(g)ΔG°=−229kJ/mo lrxn
(d) Show how a combination of reaction 1 and reaction 3 can be used to produce reaction 2.
(e) Determine the value of ΔG° for reaction 2.
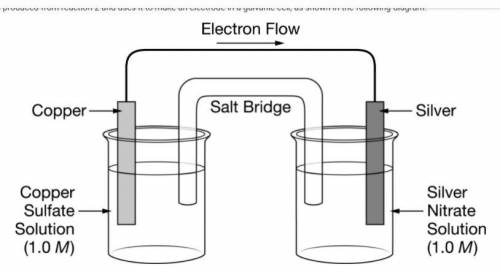
The chemist takes the Cu(s) produced from reaction 2 and uses it to make an electrode in a galvanic cell, as shown in the following diagram.
The figure presents a diagram of a galvanic cell consisting of two solutions in beakers, two metal electrodes, a salt bridge, and a wire. The half-cell on the left shows a copper electrode partially submerged in a solution of 1.0 molar copper sulfate. The half-cell on the right shows a silver electrode partially submerged in a solution of 1.0 molar silver nitrate. A salt bridge connects the two solutions. A wire connects the copper electrode and the silver electrode, and an arrow indicates that the electron flow through the wire is from the copper to the silver.
(f) Using the reduction half-reactions in the table below, write a balanced net-ionic equation representing the overall reaction that takes place as the cell operates.
Half-Reaction E° (volts)
Cu2+(aq)+2e−→Cu(s) 0.337
Ag+(aq)+e−→Ag(s) 0.800
(g) Determine the value of E° for the cell.
(h) Determine the value of ΔG° for the cell reaction.

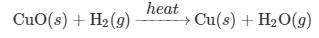



Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:30, hdhtvthjr
Which of the following natural processes is most likely to support the formation of an underwater sinkhole? a pollution buildup from deposited minerals b limestone cave collapsing due to changes in sea level c erosion of large amounts of sand moved by ocean waves d oxidation of rock formed by chemical weathering
Answers: 1

Chemistry, 22.06.2019 22:30, robertss403
How many moles of kci are produced from 2.50 moles k
Answers: 1

Chemistry, 23.06.2019 00:30, tateandvioletAHS14AY
How many moles of co2 are produced during the complete combustion of 3.6 moles of c2h6
Answers: 1
Do you know the correct answer?
Questions in other subjects:
















