.
Given the equation
2A(g) - 2B(g) + C(g)
the equilibrium constant is 0.0112 at 140°C....

Chemistry, 16.06.2021 22:20, emilyphillips1681
.
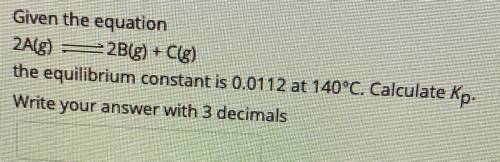
Given the equation
2A(g) - 2B(g) + C(g)
the equilibrium constant is 0.0112 at 140°C. Calculate Kp.
Write your answer with 3 decimals

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:30, lorrainelopez
The three types is stress that act on earths rocks are compression, tension, and
Answers: 1

Chemistry, 22.06.2019 23:00, tovarclaudia055
What does a numerical subscript following an element in a chemical formula mean?
Answers: 1

Chemistry, 23.06.2019 00:00, glocurlsprinces
(04.05 hc) analyze the given diagram of the carbon cycle below. part 1: which compound does c represent? part 2: name a process that could release this compound into the air. part 3: explain how the elements that form it are conserved during the carbon cycle. use complete sentences to explain your answer. justify how this compound was created from a recycling of carbon in the carbon cycle. use complete sentences to explain your answer.
Answers: 3
Do you know the correct answer?
Questions in other subjects:



Mathematics, 14.07.2019 02:00







Mathematics, 14.07.2019 02:00






