
Chemistry, 16.06.2021 22:20, erica11223344
Apply the law of mass action to determine the equilibrium expression for
2NO2Cl(aq) =2NO2 (aq) + Cl2 (aq)
OA. K=[NO_ Cl]?[NO2]?[ciz]
[NO_C1]
[NO2]?[Ciz]
OB, K=
Ock=
2[NO_IC12]
2[NO2C]
OD. K=
2[NO2Cl]
2[NO2][Ciz]
OEK=
[NO2][C12]
[NO_C1]?
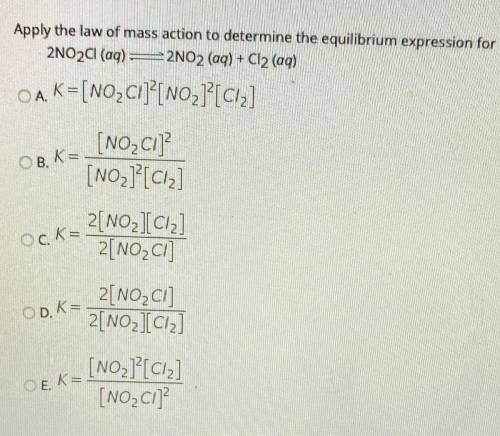

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 16:50, struckedblazing
Answer asap need it by wednesday morning calculate the ph of 0.02m hcl best answer will be brainliest
Answers: 1

Chemistry, 22.06.2019 20:30, Schoolworkspace453
Consider the following unbalanced equation for the combustion of hexane: αc6h14(g)+βo2(g)→γco2(g)+δh2o(g) part a balance the equation. give your answer as an ordered set of numbers α, β, γ, use the least possible integers for the coefficients. α α , β, γ, δ = nothing request answer part b determine how many moles of o2 are required to react completely with 5.6 moles c6h14. express your answer using two significant figures. n n = nothing mol request answer provide feedback
Answers: 2

Chemistry, 23.06.2019 01:00, birdman2540
Which of the following is in the lanthanide family? a) uranium b) promethium c) silver d) gold
Answers: 2
Do you know the correct answer?
Apply the law of mass action to determine the equilibrium expression for
2NO2Cl(aq) =2NO2 (aq) + Cl...
Questions in other subjects:


Mathematics, 15.12.2020 21:30



Mathematics, 15.12.2020 21:30

Mathematics, 15.12.2020 21:30

Mathematics, 15.12.2020 21:30

Mathematics, 15.12.2020 21:30


Mathematics, 15.12.2020 21:30






