
Chemistry, 11.10.2019 19:30, rowdycar313p0ao5k
What is the theoretical yield of ammonia that can be obtained from the reaction of 10.0 g of h2 and excess n2?
n2 + 3h2 → 2nh3
a. 90.0 g
b. 97.1 g
c. 56.3 g
d. 48.6 g
e. 28.4 g

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 13:00, torigirl4126
In what environment would mineral formation caused by high pressures and high temperatures most likely occur?
Answers: 3


Chemistry, 22.06.2019 19:30, 2020sanchezyiczela
Draw the lewis structure for the trisulfur s3 molecule. be sure to include all resonance structures that satisfy the octet rule.
Answers: 3
Do you know the correct answer?
What is the theoretical yield of ammonia that can be obtained from the reaction of 10.0 g of h2 and...
Questions in other subjects:

History, 17.11.2020 03:20

History, 17.11.2020 03:20



Spanish, 17.11.2020 03:30



Mathematics, 17.11.2020 03:30

Mathematics, 17.11.2020 03:30

Biology, 17.11.2020 03:30


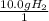 ×
×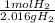 ×
×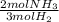 ×
×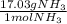 =56.3gNH↓3
=56.3gNH↓3



