Chemistry, 15.06.2021 14:00, msladycie8831
Glycerol. C3HgO3, is a substance used extensively in the manufacture of cosmetics, foodstuffs, antifreeze, and plastics. Glycerol is a water-soluble liquid
with a density of 1.2656 g/mL at 15 °C. Calculate the molarity of a solution of glycerol made by dissolving 50.000 mL glycerol at 15 °C in enough water to
make 250.00 mL of solution. The molecular weight of C3HgO3 is 92.094 amu.
O A 0.6871
O B. 3.600
O C. 63.28
O 0.92.10
O E. 2.749

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, smartie80
Transportation is the largest single source of air pollution in the united states. air pollution can harm the environment and human health. which technology could offer a solution to this problem? mufflers that reduce noise motors that run on electricity tires that improve gas mileage
Answers: 3

Chemistry, 22.06.2019 08:00, PrincessKeliah5538
Me i dont know what to do! the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1

Chemistry, 22.06.2019 13:00, jaylanmahone223
6. using 3 – 4 sentences explain (in your own words) why water expands when it freezes? 7. using your knowledge of colligative properties explain whether sodium chloride or calcium chloride would be a more effective substance to melt the ice on a slick sidewalk. use 3 – 4 sentences in your explanation.
Answers: 1
Do you know the correct answer?
Glycerol. C3HgO3, is a substance used extensively in the manufacture of cosmetics, foodstuffs, antif...
Questions in other subjects:

Geography, 30.08.2019 21:40




History, 30.08.2019 21:40


Mathematics, 30.08.2019 21:40




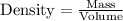 ......(1)
......(1)
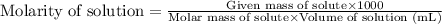 .....(2)
.....(2)