
Chemistry, 14.06.2021 22:40, jazminemendezhidalgo
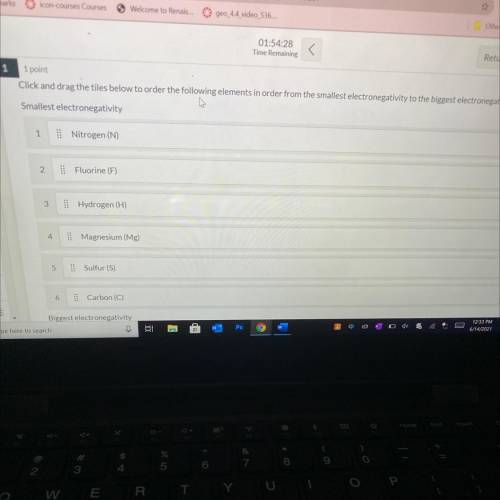
Drag the tiles below to order the following elements in order from the smallest electronegativity to the biggest electron
electronegativity
Nitrogen (N)
Fluorine (F)
Hydrogen (H)
:
Magnesium (Mg)
Sulfur (S)
Carbon (C)

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:50, vanessa051266
In an exothermic reaction the bonding energy of the product is: less than the reactants same as the reactants greater than the reactants dependent upon the presence of a catalyst
Answers: 1


Chemistry, 22.06.2019 12:00, Alexislol7908
From the options provided for each element below, choose the properties that it may have based on its location in the periodic table fluorine (f): highly reactive nonmetal shiny a conductor
Answers: 1
Do you know the correct answer?
Drag the tiles below to order the following elements in order from the smallest electronegativity to...
Questions in other subjects:

Computers and Technology, 18.10.2021 07:40

Mathematics, 18.10.2021 07:40

Computers and Technology, 18.10.2021 07:40

Mathematics, 18.10.2021 07:40

English, 18.10.2021 07:40


Mathematics, 18.10.2021 07:40

Geography, 18.10.2021 07:40

Mathematics, 18.10.2021 07:40

English, 18.10.2021 07:40






