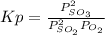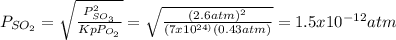Consider the following reaction at 298 K.
2 SO2(g) + O2(g) → 2 SO3(g)
An equilibrium mixture...

Chemistry, 14.06.2021 19:40, hihudgins902
Consider the following reaction at 298 K.
2 SO2(g) + O2(g) → 2 SO3(g)
An equilibrium mixture contains O2(g) and SO3(g) at partial pressures of 0.43 atm and 2.6 atm, respectively. Using data from Appendix 4, determine the equilibrium partial pressure of SO2 in the mixture.
atm.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:30, xojade
Which statements are true concerning mineral formation? check all that apply. the slower the cooling, the larger the crystals. the faster the cooling, the smaller the crystals. crystals formed from magma are smaller than crystals formed from lava. minerals can only form in solutions when the solution is heated deep underground. when a solution cools, elements and compounds leave the solution and crystallize as minerals. minerals formed from hot water solutions can form narrow channels in the surrounding rock.
Answers: 1

Chemistry, 22.06.2019 17:30, llamasking
Observation and experimentation have led many scientists to accept a theory about the origin of the universe. this theory is called the big bang theory. scientific evidence collected and observed by scientists around the world suggests that the universe is ever expanding from a hot and dense initial state. what makes this a scientific theory? (2 points)
Answers: 2

Chemistry, 22.06.2019 23:30, hcllxxhhlpcj
Rank substituents in order of their priority when assigning the e or z label to an alkene. i, ch2i , h, ch2ch2cl, f
Answers: 2

Chemistry, 23.06.2019 08:00, colbygreen6189
Identify the decay mode particle emitted from the th 234
Answers: 1
Do you know the correct answer?
Questions in other subjects:





Mathematics, 12.03.2020 06:04



Spanish, 12.03.2020 06:04