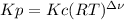Chemistry, 14.06.2021 17:00, therealrg10
Equilibrium constants for gases can be expressed in terms of concentrations, Kc, or in terms of partial pressures, Kp. Which one of the following statements regarding Kc and Kp is correct?
a. Kc and Kp are equal when all stoichiometric coefficients in the balanced reaction equation equal one.
b. Kc and Kp are equal when the conditions are standard (P= 1 atm, T=298 K)
c. Kc and Kp are equal when the sum of the stoichiometric coefficients for the products equals the sum of the stoichiometric coefficients for the reactants.
d. Kc and Kp can never be equal.
e. Kc and Kp have the same values but different units.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:00, shifaxoxoxo
What term is applied to a scientist who studies ancient life, including animal and plant fossils a. anthropologist b. dendroclimatologist c. geophysicist d. paleontologist
Answers: 2

Chemistry, 22.06.2019 12:00, 1963038660
What are the first two quantum numbers for the electrons located in subshell 4d? what are the first three quantum numbers for the electrons located in subshell 2s? how many electrons can be held in a sublevel l = 3? how many electrons can be held in the energy level n = 4? how many electrons in an atom can share the quantum numbers n = 4 and l = 3?
Answers: 1

Chemistry, 22.06.2019 17:00, destinyycooper
What is the approximate vapor pressure when the gas condenses at 70 degrees celsius
Answers: 2
Do you know the correct answer?
Equilibrium constants for gases can be expressed in terms of concentrations, Kc, or in terms of part...
Questions in other subjects:






Mathematics, 14.06.2020 01:57