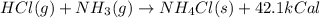Chemistry, 12.06.2021 23:40, kenisonpaigebosma
When HCl(g) reacts with NH3(g) to form NH4Cl(s), 42.1 kcal of energy are evolved for each mole of HCl(g) that reacts. Write a balanced equation for the reaction with an energy term in kcal as part of the equation.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, momof7hardings
When would a bouncy ball have the most potential energy
Answers: 2



Chemistry, 22.06.2019 17:00, destinyycooper
What is the approximate vapor pressure when the gas condenses at 70 degrees celsius
Answers: 2
Do you know the correct answer?
When HCl(g) reacts with NH3(g) to form NH4Cl(s), 42.1 kcal of energy are evolved for each mole of HC...
Questions in other subjects:




History, 10.02.2020 23:50