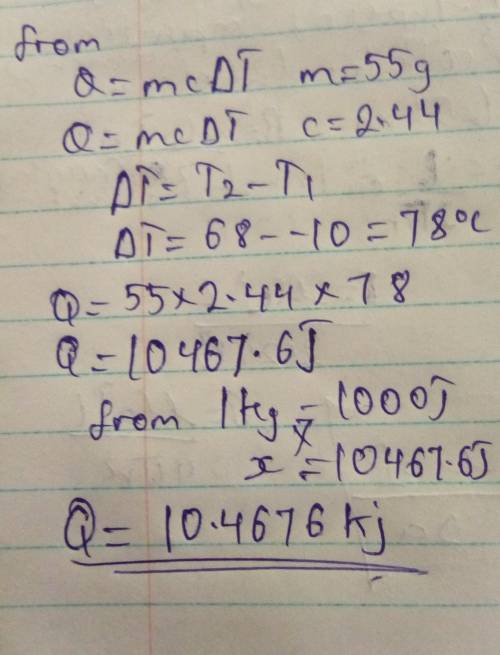Chemistry, 11.06.2021 14:00, kennethDA19
The specific heat of ethanol is 2.44 J/(g °C). How many kilojoules of energy are required to heat 55.0 g of ethanol from -10.0°C to 68.0°C

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 23.06.2019 00:30, evelynalper08
Which radioisotope is used to date fossils? a. oxygen-16 b. carbon-14 c. uranium-238 d. carbon-12
Answers: 2

Chemistry, 23.06.2019 01:30, sheldonwaid4278
Magnesium is the limiting reactant in this experiment. calculate the theoretical yield of mgo for each trial. trial 1: trial 2: data mass of empty crucible with lid trial 1: 26.688 trial 2: 26.681 mass of mg metal, crucible, and lid trial 1: 26.994 trial: 2 26.985 mass of mgo, crucible, and lid trial 1: 27.188 trial 2: 27.180
Answers: 1
Do you know the correct answer?
The specific heat of ethanol is 2.44 J/(g °C). How many kilojoules of energy are required to heat 55...
Questions in other subjects:


Mathematics, 28.07.2019 13:30

Social Studies, 28.07.2019 13:30

Social Studies, 28.07.2019 13:30

Mathematics, 28.07.2019 13:30


Mathematics, 28.07.2019 13:30



Mathematics, 28.07.2019 13:30