
Chemistry, 10.06.2021 23:20, jonathon3957
(Picture Included) A handbook lists the density of lead as 11.3 g/mL. Several groups of students are attempting to determine the density of a lead weight by various methods. Calculate the average density measured by each group, and the percentage error in each average.
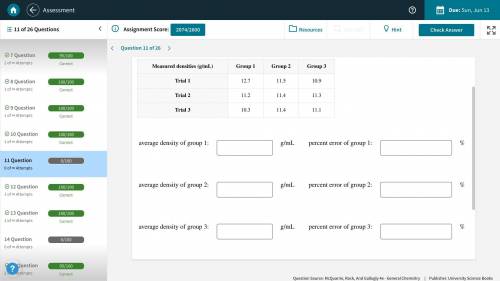

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 18:00, meowmeowcow
Find the mass, in grams, of 5.00*10^23 molecules of f2
Answers: 3

Chemistry, 22.06.2019 22:30, teagan56
Gusing the milligrams of ascorbic acid you entered above, the ratio of total sample volume to aliquot volume, and the total milligrams of the vitamin c tablet that you dissolved, calculate the mass of ascorbic acid in the vitamin c tablet for each trial. do this by scaling up to find the amount (mg) of ascorbic acid in your 250 ml flask. enter your calculated mass of ascorbic acid in the vitamin c tablet, for each trial. be sure to enter your calculated mass in the corresponding order that you entered your milligrams of ascorbic acid. the milligrams of ascorbic acid you entered for entry #1 previously should correspond to the mass of ascorbic acid that you enter for entry #1 here.
Answers: 1

Chemistry, 23.06.2019 04:20, monifaWilson
The graph shows one consequence of urban sprawl. how did urban sprawl contribute to the change in biodiversity
Answers: 2
Do you know the correct answer?
(Picture Included) A handbook lists the density of lead as 11.3 g/mL. Several groups of students are...
Questions in other subjects:


Social Studies, 12.11.2019 00:31

Biology, 12.11.2019 00:31



Arts, 12.11.2019 00:31


English, 12.11.2019 00:31

History, 12.11.2019 00:31

Mathematics, 12.11.2019 00:31






