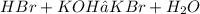Chemistry, 10.06.2021 23:10, jocelynfray16
Assume that the reaction of aqueous hydrobromic acid solution and potassium hydroxide base undergoes a complete neutralization reaction.
a. Write a balanced chemical equation.
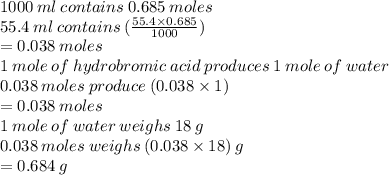
b. How many grams of water can be produce from the complete reaction of excess hydrobromic acid and 55.4 mL of 0.685 M potassium hydroxide solution, assuming that potassium hydroxide is the limiting reactant?
A solution of hydrobromic acid is formed by dissolving 5.00 grams in enough water to make 1.5 L solution.
c.. What was the molarity of this solution?
d. What is the pH of this solution?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, ayoismeisjjjjuan
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 1

Chemistry, 22.06.2019 13:50, aesthetickait
How does the motion of particles in a gas change as the gas cools
Answers: 2

Chemistry, 22.06.2019 18:00, jeepjose58
What amount of heat is exchanged when 106.2 grams of substance y goes from a liquid at 35 degrees celsius to a solid at the same temperature? melting point of substance y = 35 degrees c; δhvaporization = 3.67 j/mol; δhfusion = 3.30 j/mol. mwsubstance y = 28.22 g/mol. −12.4 j −3.51 x 102 j 1.24 x 101 j 351 j
Answers: 1

Chemistry, 22.06.2019 22:30, lanashanabJHsbd1099
Who discovered a pattern to the elements in 1869?
Answers: 1
Do you know the correct answer?
Assume that the reaction of aqueous hydrobromic acid solution and potassium hydroxide base undergoes...
Questions in other subjects:

English, 10.11.2021 19:50




Mathematics, 10.11.2021 19:50



Mathematics, 10.11.2021 19:50


History, 10.11.2021 19:50