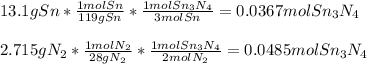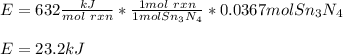How much energy is produced when 13.1 g of tin reacts with 2.715 g of N2 ?
I.
3 Sn + 2 N2Sn3N...

Chemistry, 09.06.2021 07:50, matthewlucas8613
How much energy is produced when 13.1 g of tin reacts with 2.715 g of N2 ?
I.
3 Sn + 2 N2Sn3N4 + 632 KJ
Hint change grams to moles first.
1 mole Sn= 119g
1 mole N2= 28 g

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 18:10, NEONREDBLADE
Areader can tell that the meaning of “obnoxious” will include “having the quality of something” because of the .a) prefix b)pronunciation c)suffix d) word root
Answers: 3


Chemistry, 22.06.2019 20:30, jaydenbrock
Identify the correct mole ratio for each substance. sodium chloride (nacl) na: cl = 1: ammonium nitrate (nhno) h: o = 4:
Answers: 1
Do you know the correct answer?
Questions in other subjects: