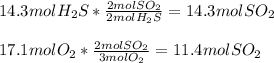Chemistry, 09.06.2021 07:50, Miloflippin7339
How much energy is used when 14.3 moles of hydrosulfuric acid reacts with 17.1 moles
of oxygen?
2 H2S + 3 02 + 175 KJ
—->2 SO2 + 2 H20
Which substance is the limiting reactant?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 17:30, mwest200316
To find the enthalpy of a reaction in the lab, you measured the of the reactants and the change during the reaction.
Answers: 1

Chemistry, 22.06.2019 21:00, Janznznz4012
Once similarity and one difference between a mixture of elements and a mixture of compounds
Answers: 3

Chemistry, 23.06.2019 03:50, mobslayer88
How many moles of potassium are needed to react completely with 12.8 moles of magnessium bromide?
Answers: 2

Chemistry, 23.06.2019 04:00, onegirl435
The movement of tectonic plates and in two locations is described below: location a: tectonic played push together location b: tectonic plates push apart
Answers: 1
Do you know the correct answer?
How much energy is used when 14.3 moles of hydrosulfuric acid reacts with 17.1 moles
of oxygen?
Questions in other subjects:


History, 29.04.2021 18:50

English, 29.04.2021 18:50

Mathematics, 29.04.2021 18:50





Chemistry, 29.04.2021 18:50

Mathematics, 29.04.2021 18:50