Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, logan12345677885675
What are the primary responsibilities of a chemical engineer involved in "r& d"? develop large scale manufacturing operations discover new products and processes training of new chemists determine products needed by consumers
Answers: 2

Chemistry, 22.06.2019 14:00, coylenoah0
How many absorptions would you expect to observe in the 13c nmr spectra of the following molecules? a) 3-chloropentane b) cis-4-methyl-2-pentene
Answers: 2


Chemistry, 22.06.2019 19:50, mikaylaaaaa
If a gas has an initial pressure of 101kpa and a volume of 10l, then it expands to a volume of 20l, what is the new pressure?
Answers: 2
Do you know the correct answer?
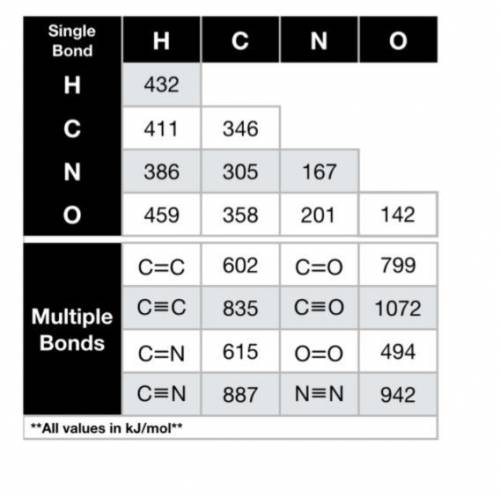
Using the bond energies provided, calculate the enthalpy of the reaction (∆Hrxn, in kJ) for the comb...
Questions in other subjects:

Mathematics, 11.05.2021 01:40



Mathematics, 11.05.2021 01:40




Mathematics, 11.05.2021 01:40


Mathematics, 11.05.2021 01:40