
Chemistry, 08.06.2021 23:20, lorriegoodman3483
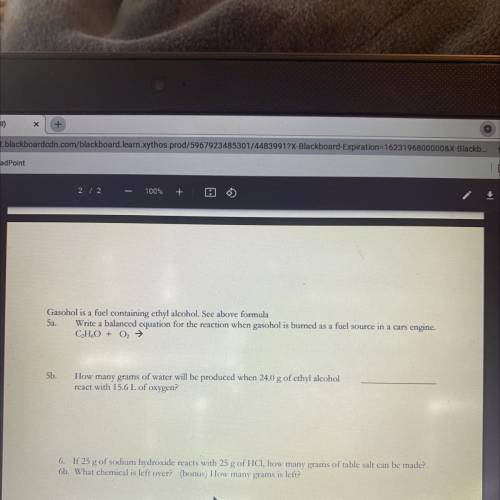
Gasohol is a fuel containing ethyl alcohol. See above formula
5a. Write a balanced equation for the reaction when gasohol is burned as a fuel source in a cars engine.
C2H6O + O2 →
5b.
How many grams of water will be produced when 24.0 g of ethyl alcohol
react with 15.6 L of oxygen?

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 03:20, Richwave17
Which type of substance ionizes partially and gives off hydrogen ions when dissolved in water? a. strong acid b. strong base c. weak acid d. weak base
Answers: 1

Chemistry, 22.06.2019 09:10, cheesedoodle
When a nucleus absorbs a neutron and then breaks apart, there are many products of the reaction. what is not a product of a nuclear fission reaction
Answers: 1

Chemistry, 22.06.2019 11:50, hadwell34
Which of the following statements about hybrid orbitals is or are true? choose all that apply. choose all that apply. under sp2 hybridization, the large lobes point to the vertices of an equilateral triangle. after an atom undergoes sp hybridization there is one unhybridized p orbital on the atom. the angle between the large lobes of sp3 hybrids is 109.5∘
Answers: 2
Do you know the correct answer?
Gasohol is a fuel containing ethyl alcohol. See above formula
5a. Write a balanced equation for the...
Questions in other subjects:




Physics, 03.08.2019 13:00






Spanish, 03.08.2019 13:00






