Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:50, sgslayerkingminecraf
Which of the following statements about acidic water is true? a. acid has no effect on the h, o molecules. b. the solution contains a larger number of oh ions than h, o ions. c. the solution contains a larger number of h, o ions than qh ions. d. the solution contains an equal number of h, o ions and oh ions. none of the above e.
Answers: 1



Chemistry, 22.06.2019 20:30, ashley4329
Select all the correct answers. which compounds have the empirical formula ch20? (multiple answers)a. c2h4o2b. c3h603c. ch2o2d. c5h1005e. c6h1206
Answers: 2
Do you know the correct answer?
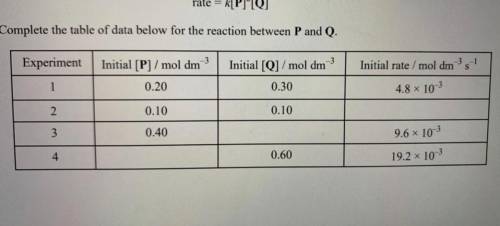
The initial rate of the reaction between substances P and Q was measured in a series of
experiments...
Questions in other subjects:

Chemistry, 20.11.2020 15:40


History, 20.11.2020 15:40

English, 20.11.2020 15:40

Biology, 20.11.2020 15:40

Biology, 20.11.2020 15:40

English, 20.11.2020 15:50



Mathematics, 20.11.2020 15:50