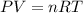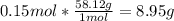Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 13:50, kelonmazon2492
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3

Do you know the correct answer?
A) If we have a 4.5 L container of CH 10 gas at a temperature of 178 K and a pressure of 0.50 atm, t...
Questions in other subjects:



Mathematics, 22.08.2019 18:40

Spanish, 22.08.2019 18:40

English, 22.08.2019 18:40


Mathematics, 22.08.2019 18:40

History, 22.08.2019 18:40

Mathematics, 22.08.2019 18:40

Chemistry, 22.08.2019 18:40