
Chemistry, 08.06.2021 03:20, avisconti571
A sample of 0.2140 g of an unkown substance monoprotic acid was dissolved in 25.0 mL of water and titrated with 0.950 M NaOH. The acid required 27.4 mL of base to reach the equivalence point. After 15.0 mL of base had been added in the titration, the pH was found to be 6.50. What is the Ka for the unknown acid?

Answers: 3
Other questions on the subject: Chemistry




Chemistry, 23.06.2019 16:40, raincalderxn
Which statement is true about market economies? government goals drive business decisions. people have the freedom to choose their jobs. several are market economies vist around the world
Answers: 2
Do you know the correct answer?
A sample of 0.2140 g of an unkown substance monoprotic acid was dissolved in 25.0 mL of water and ti...
Questions in other subjects:

English, 13.01.2021 17:40




Biology, 13.01.2021 17:40

Social Studies, 13.01.2021 17:40

Social Studies, 13.01.2021 17:40





 of HA
of HA
![K_a=\frac{[A^-].[H^+]}{[HA]}](/tpl/images/1366/2932/3c83d.png)
![$[HA] = \frac{^nH_A}{V}$](/tpl/images/1366/2932/b0ec6.png)

![$[NaOH]= \frac{0.015L \times 0.0950 M}{V}$](/tpl/images/1366/2932/a5013.png)


 and 0.0356 M
and 0.0356 M 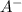
![[H^+]](/tpl/images/1366/2932/07acb.png)
![$[H^+] = 10^{-pH}$](/tpl/images/1366/2932/d6eff.png)
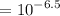
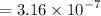


 .
.




