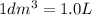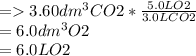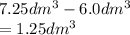Chemistry, 04.06.2021 20:20, juliannabartra
Propane burns to form carbon dioxide and water. The equation for the reaction is:
C 3 H 8 (g)+5 O 2 (g) 3 CO 2 (g)+4 H 2 O(l)
3.60d * m ^ 3 carbon dioxide is produced when a sample of propane is burned in 7.25d * m ^ 3 oxygen.
Calculate the volume of unreacted oxygen. Give your answer in cm^ 3

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, babygirl2984
There is an area in idaho named craters of the moon where most of the ground is covered with basalt, adark gray, igneous rock with no visibl crystals. what can you infer about the geographical history of the area?
Answers: 1


Chemistry, 22.06.2019 14:00, IdkHowToDoMath
What term describes technology that operates on an atomic level
Answers: 2
Do you know the correct answer?
Propane burns to form carbon dioxide and water. The equation for the reaction is:
C 3 H 8 (g)+5 O 2...
Questions in other subjects:

Health, 24.09.2021 14:00

Biology, 24.09.2021 14:00



Mathematics, 24.09.2021 14:00

English, 24.09.2021 14:00