
Chemistry, 04.06.2021 18:00, gissellesolorza
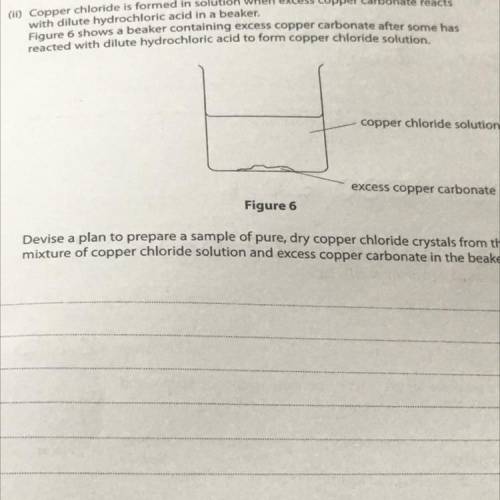
(11) Copper chloride is formed in solution when excess copper carbonate reacts
with dilute hydrochloric acid in a beaker.
Figure 6 shows a beaker containing excess copper carbonate after some has
reacted with dilute hydrochloric acid to form copper chloride solution.
DO NOT WRITE IN THIS AREA
DO NOT WRITE IN THIS AREN
copper chloride solution
excess copper carbonate
Figure 6
Devise a plan to prepare a sample of pure, dry copper chloride crystals from the
mixture of copper chloride solution and excess copper carbonate in the beaker.
DO NOT
THIS AREA
A

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, jamesnaquan132
What is the most stable monatomic ion formed from nitrogen
Answers: 2

Chemistry, 22.06.2019 14:50, wcraig1998
Complete the following statements to describe solids, liquids, and gases. select the correct answer from each drop-down menu. a solid a definite volume and a definite shape. a liquid a definite volume and a definite shape. a gas a definite volume and a definite shape
Answers: 1

Chemistry, 23.06.2019 01:00, daniel1480
Which fossil fuel is mainly used for heating and cooking? a. electricity b. coal c. petroleum d. natural gas
Answers: 2
Do you know the correct answer?
(11) Copper chloride is formed in solution when excess copper carbonate reacts
with dilute hydrochl...
Questions in other subjects:

Health, 04.04.2021 22:40




English, 04.04.2021 22:40

Physics, 04.04.2021 22:40


Mathematics, 04.04.2021 22:40

Business, 04.04.2021 22:40

History, 04.04.2021 22:40






