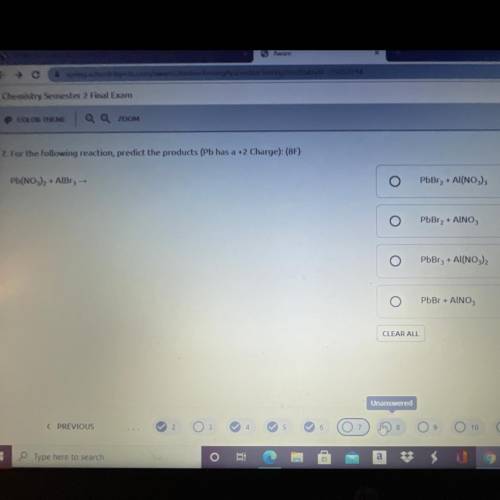
Pb(NO3)2 + AlBr3 →


Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:30, perezanthony2403
Which describes fat? a: a carbohydrate that produces energy b: a nucleic acid that directs cell function c: a lipid that stores energy d: a protein that speeds up a chemical reaction
Answers: 1

Chemistry, 22.06.2019 18:00, sandeebassett3
Mercury turns to vapor at 629.88 k how much heat is lost 175 g of mercury vapor at 650 current condenses to a liquid at 297 ca mercury turns to weber at 629.88 kelvin how much he is lost 175 g of mercury vapor and 650 coming condensers to liquidate 297 kevin
Answers: 2

Chemistry, 22.06.2019 19:50, jakaylathomas11
A2.5% (by mass) solution concentration signifies that there is a 2.5 % (by mass) solution concentration signifies that there is blank of solute in every 100 g of solution. of solute in every 100 g of solution
Answers: 3
Do you know the correct answer?
For the following reaction, predict the products (Pb has a +2 Charge):
Pb(NO3)2 + AlBr3 →
Pb(NO3)2 + AlBr3 →
Questions in other subjects:


Mathematics, 12.05.2021 04:00

Geography, 12.05.2021 04:00



Engineering, 12.05.2021 04:00


Mathematics, 12.05.2021 04:00

Mathematics, 12.05.2021 04:00