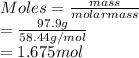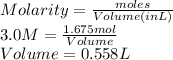PLEASE HELP Let’s say a colleague working in the lab needs to create a solution containing 97.9 grams of NaCl. If she has a 3.0 M stock solution of NaCl dissolved in water, how many liters of the stock solution would she need to have 97.9 grams NaCl? Remember the molar mass of NaCl is 58.44 g/mol.

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 16:50, briansalazar17
Answer asap need by wednesday morning calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 best answer will be brainliest
Answers: 3
Do you know the correct answer?
PLEASE HELP
Let’s say a colleague working in the lab needs to create a solution containing 97.9 gra...
Questions in other subjects: