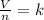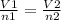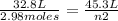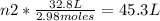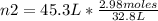Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:30, nique0808
What are the major products produced in the combustion of c10h22 under the following conditions? write balanced chemical equations for each. a. an excess of oxygen b. a slightly limited oxygen supply c. a very limited supply of oxygen d. the compound is burned in air
Answers: 2

Chemistry, 22.06.2019 02:10, fvmousdiana
Determine the percent sulfuric acid by mass of a 1.61 m aqueous solution of h2so4. %
Answers: 2


Chemistry, 22.06.2019 10:10, andersonemma2222
How do you identify the anode on a power source such as a battery? how do you identify the cathode? how are terms anion and cation?
Answers: 1
Do you know the correct answer?
2.98 moles of H2 at 35°C and 2.3 atm are in a 32.8 L container. How many moles of H2 are in a 45.3 L...
Questions in other subjects:



Mathematics, 03.06.2021 17:10

Mathematics, 03.06.2021 17:10

Spanish, 03.06.2021 17:10


Chemistry, 03.06.2021 17:10



Mathematics, 03.06.2021 17:20