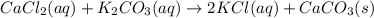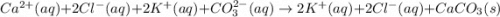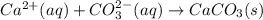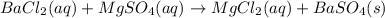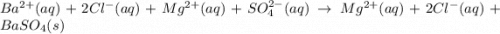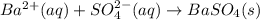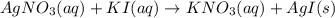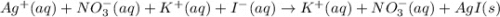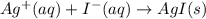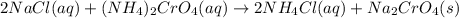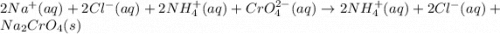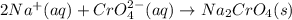Chemistry, 03.06.2021 23:40, mirandac6775
Show the complete ionic equation and net ionic equation for all the equations below, then state whether or not a precipitate (insoluble compound) will form. To receive full credit, you must show ALL your work.
Cacl2(aq) + K2co3(aq) + >
Bacl2(aq) + MgSO4(aq) + >
AgNO3(aq) + Kl(aq) →
Nacl(aq) + (NH4)2Cro4(aq) →

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:20, dgadam7495
Calculate the molarity of 48.0 ml of 6.00 m h2so4 diluted to 0.250 l .
Answers: 1

Chemistry, 22.06.2019 08:00, mariamakonteh31
Will give ! what are the advantages and disadvantages of nuclear power? check all that apply. one advantage of nuclear energy is that it does not produce carbon dioxide emissions. storage of nuclear waste is a short-term problem associated with nuclear energy. the problem with uranium mining is that a large quantity of uranium must be extracted to meet energy needs because the energy release from uranium fission is so low. safe operation of a nuclear power plant can be jeopardized by a human mistake.
Answers: 1

Chemistry, 22.06.2019 13:00, jaylanmahone223
6. using 3 – 4 sentences explain (in your own words) why water expands when it freezes? 7. using your knowledge of colligative properties explain whether sodium chloride or calcium chloride would be a more effective substance to melt the ice on a slick sidewalk. use 3 – 4 sentences in your explanation.
Answers: 1
Do you know the correct answer?
Show the complete ionic equation and net ionic equation for all the equations below, then state whet...
Questions in other subjects:

Mathematics, 20.08.2019 16:30

History, 20.08.2019 16:30

Mathematics, 20.08.2019 16:30

Mathematics, 20.08.2019 16:30


Social Studies, 20.08.2019 16:30




History, 20.08.2019 16:30