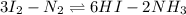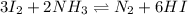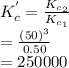Chemistry, 03.06.2021 21:40, scottytohotty
A mixture containing nitrogen, hydrogen, and iodine established the following equilibrium at 400 °C:2NH3(g) + 3I2(g) ⇌ N2(g) + 6HI(g)Use the information below to calculate Kc for this reaction. N2(g)+3H2(g)⇌2NH3(g) Kc1= 0.50 at 400CH2(g)+I2(g)⇌2HI(g Kc2= 50 at 400°C

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:10, kellinvagneur
Which electron configuration represents the electrons in an atom of sodium in the ground state at stp
Answers: 1

Chemistry, 22.06.2019 13:30, amandajbrewerdavis
Table sugar completely dissolved in water is an example of a?
Answers: 1


Chemistry, 22.06.2019 20:20, catchonyet
Which formula equation represents the burning of sulfur to produce sulfur dioxide? s(s) + o2(g) 4502(9) 2h2s(s) + 302(g) —> 2h20(0) + 2502(9) 4fes2+1102 —> 2fe2o3 + 8502 2802(g) + o2(9) v205 , 2503(9)
Answers: 1
Do you know the correct answer?
A mixture containing nitrogen, hydrogen, and iodine established the following equilibrium at 400 °C:...
Questions in other subjects:


Spanish, 01.09.2019 21:50




Mathematics, 01.09.2019 21:50


Mathematics, 01.09.2019 21:50

Social Studies, 01.09.2019 21:50


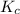 for this reaction is 250000.
for this reaction is 250000.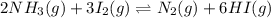
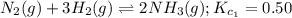 ... (1)
... (1)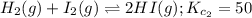 ... (2)
... (2)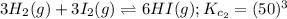 ... (3)
... (3)