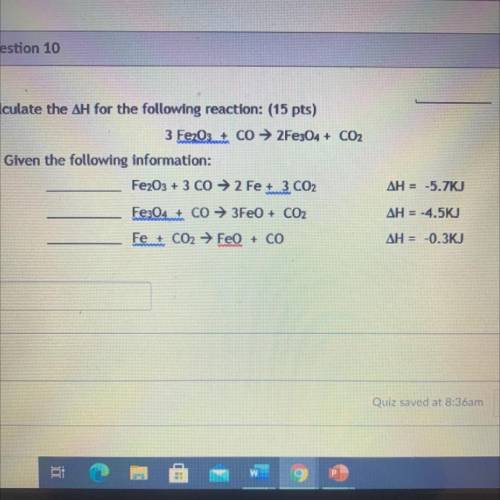
Calculate the ΔH for the following reaction: (15 pts)
3 Fe2O3+ Co → 2Fe304 + CO2
Given the fo...

Chemistry, 03.06.2021 17:00, hjeffrey168
Calculate the ΔH for the following reaction: (15 pts)
3 Fe2O3+ Co → 2Fe304 + CO2
Given the following information:
Fe2O3 + 3 CO → 2 Fe 3 CO2
ΔH = -5.7KJ
Fe3O4 + CO → 3FeO + CO2
ΔH = -4.5KJ
Fe + CO2 → FeO + CO
ΔH = -0.3KJ

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 17:00, smelcher3900
According to the kinetic-molecular theory, what happens to a liquid when it is transferred from one container to another? the volume and the shape stay the same. the volume increases to fill the new container, but the shape stays the same. the volume stays the same, but the shape changes to fit the new container. the volume and the shape change to fill the new container.
Answers: 2

Chemistry, 22.06.2019 22:30, darkshaders11
Is the idea of spontaneous generation supported by redi's experiment? justify your answer in 2-3 sentences?
Answers: 1


Chemistry, 23.06.2019 09:50, jay4881
T(s) in2os] (m) 0 185 2.39 546 1.90 725 1.70 the decomposition of n205 can be described by the equation 2.68 given these data for the reaction at 45°c in carbon tetrachloride solution, calculate the average rate of reaction for each successive time interval. ntr s to 185 s 185 s to 546 s 546 s to 725 s number number number reaction rate: m/s m/s m/s
Answers: 1
Do you know the correct answer?
Questions in other subjects:

History, 29.01.2020 07:53

Health, 29.01.2020 07:53

Mathematics, 29.01.2020 07:53


Social Studies, 29.01.2020 07:53

History, 29.01.2020 07:53

Geography, 29.01.2020 07:53

History, 29.01.2020 07:53


History, 29.01.2020 07:53






