
Chemistry, 03.06.2021 14:00, emilygoolsby2123
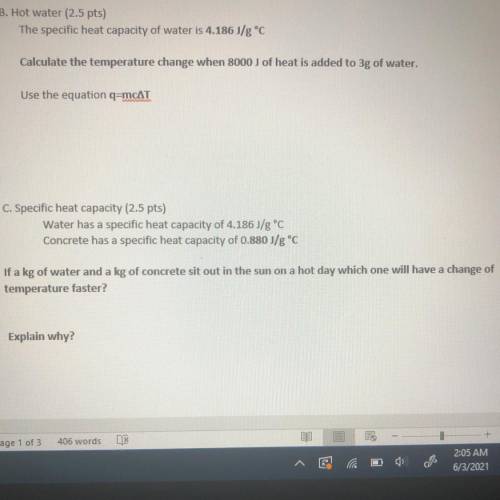
B. Hot water
The specific heat capacity of water is 4.186J/g °C
Calculate the temperature change when 8000 j of heat is added to 3g of water.
Use the equation q=mcAT
C. Specific heat capacity
Water has a specific heat capacity of 4.186J/g °C
Concrete has a specific heat capacity of 0.880 J/g °C
If a kg of water and a kg of concrete sit out in the sun on a hot day which one will have a change of temperature faster?
Explain why?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:00, daniellekennedy05
If there is any 12 to 14 girls that need a boyfriend just follow me and let me know
Answers: 1

Chemistry, 22.06.2019 21:00, rah45
Which of these is an example of pseudoscience? a) predicting the time of sunrise based on data on position of earth b) predicting the date of the moon phases based on data on position of earth c) predicting eclipses based on the position of the sun and the moon d) predicting future events in a person's life based on the position of the moon
Answers: 1
Do you know the correct answer?
B. Hot water
The specific heat capacity of water is 4.186J/g °C
Calculate the temperature cha...
Calculate the temperature cha...
Questions in other subjects:

Biology, 15.04.2021 16:20

English, 15.04.2021 16:20

Mathematics, 15.04.2021 16:20



Mathematics, 15.04.2021 16:20










