
Chemistry, 03.06.2021 05:40, jaygamer37
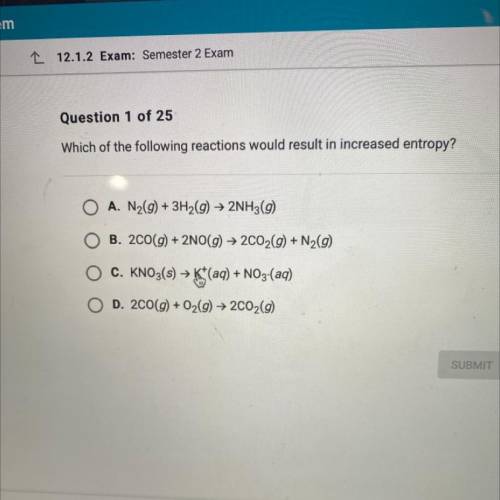
Which of the following reactions would result in increased entropy?
A. N2(g) + 3H2(g) + 2NH3(9)
B. 200(g) + 2NO(g) → 2C02(g) + N2(9)
C. KNO3(s) > K+(aq) + NO3-(aq)
D. 200(g) + O2(g) → 2C02(9)

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:30, krharris
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 2

Chemistry, 22.06.2019 10:10, babyphoraaaaa
For the reaction, 4 a(g) + 3 b(g) => 2 c(g), the following data were obtained at constant temperature. experiment initial[a],mol/l initial [b],mol/l initial rate, m/min 1 0.200 0.150 5.00 2 0.400 0.150 10.0 3 0.200 0.300 10.0 4 0.400 0.300 20.0 which of the following is the correct rate law for the reaction? 1. rate = k[a]2[b]2 2. rate = k[a][b] 3. rate = k[a]2[b] 4. rate = k[a][b]2
Answers: 3

Do you know the correct answer?
Which of the following reactions would result in increased entropy?
A. N2(g) + 3H2(g) + 2NH3(9)
Questions in other subjects:

Mathematics, 04.06.2020 20:05

English, 04.06.2020 20:06



Mathematics, 04.06.2020 20:06

Social Studies, 04.06.2020 20:06









