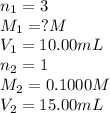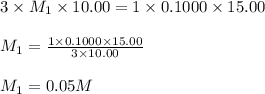Chemistry, 03.06.2021 04:30, elissiashontelbrown
g 10.00 mL of phosphoric acid (H3PO4) are titrated with 0.1000 M sodium hydroxide. 15.00 mL of the sodium hydroxide solution are used in this experiment. Determine the molarity of phosphoric acid.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:40, raquelqueengucci25
In this synthesis reaction what products will form
Answers: 1

Chemistry, 22.06.2019 10:30, freddhendrickss
When the speed of the bottle is 2 m/s, the average maximum height of the beanbag is m.
Answers: 2

Chemistry, 22.06.2019 13:50, kelonmazon2492
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3

Chemistry, 22.06.2019 14:00, jivsf
The two naturally occurring isotopes of chlorine are 35cl (34.969 amu, 75.77%) and 37cl (36.966 amu, 24.23%). the two naturally occurring isotopes of bromine are 79br (78.918 rm amu, 50.69%) and 81br (80.916 amu, 49.31%). chlorine and bromine combine to form bromine monochloride, brcl. 1. how many peaks will be present in a mass spectrum for brcl? the four combinations of molecule possible given these four isotopes are: 81br37cl, 81br35cl, 79br37cl, and 79br35cl. 2. what are the masses of the four different brcl molecules? express the masses using six significant figures, in decreasing numeric order (highest to lowest), separated by commas.
Answers: 3
Do you know the correct answer?
g 10.00 mL of phosphoric acid (H3PO4) are titrated with 0.1000 M sodium hydroxide. 15.00 mL of the s...
Questions in other subjects:


Mathematics, 05.10.2019 10:01

Mathematics, 05.10.2019 10:01

Chemistry, 05.10.2019 10:01

History, 05.10.2019 10:01






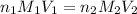 ........(1)
........(1)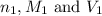 are the n-factor, molarity and volume of acid that is
are the n-factor, molarity and volume of acid that is 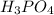
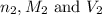 are the n-factor, molarity and volume of the base that is NaOH
are the n-factor, molarity and volume of the base that is NaOH