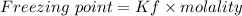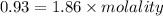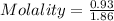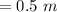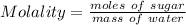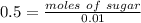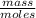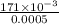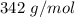Chemistry, 03.06.2021 04:20, alissalhenry
A solution prepared by dissolving 171 mg of a sugar (a molecular compound and a nonelectrolyte) in 1.00 g of water froze at -0.930°C. What is the molar mass of this sugar? The value of Kf is 1.86°C/m.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:30, cxttiemsp021
Calculate the mass of carbon in 97.0 g of sucrose c12h22o11
Answers: 3

Chemistry, 22.06.2019 16:50, brandiwingard
What is conserved in the reaction shown below? h2(g) + cl2 (g) --> 2hcl(g)a. mass onlyb. mass and moles onlyc. mass, moles, and molecules onlyd. mass, moles, molecules, and volume
Answers: 2

Chemistry, 22.06.2019 16:50, TheOriginal2x
Assuming complete dissociation of the solute, how many grams of kno3 must be added to 275 ml of water to produce a solution that freezes at -14.5 c? the freezing point for pure water is 0.0 c and k_f is equal to 1.86 c/m
Answers: 3
Do you know the correct answer?
A solution prepared by dissolving 171 mg of a sugar (a molecular compound and a nonelectrolyte) in 1...
Questions in other subjects:










History, 21.06.2019 13:30