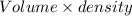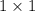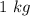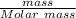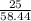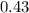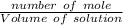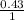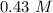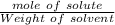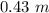Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:00, familyvazquez7
What is the temperature of 0.750 mol of a gas stored in a 6,850 ml cylinder at 2.21 atm? . 2.95 k 5.24 k 138 k 246 k
Answers: 3

Chemistry, 22.06.2019 22:00, luciaaviles3
Pls ill give u brainliest which of the following is true about science? 1. political conditions are unable to influence it. 2. economic concerns may prevent it from solving problems.
Answers: 2

Chemistry, 22.06.2019 23:50, josie311251
Be sure to answer all parts. the following equilibrium constants were determined at 1123 k: c(s) + co2(g) ⇌ 2co(g) k'p = 1.30 × 1014 co(g) + cl2(g) ⇌ cocl2(g) k''p = 6.00 × 10−3 calculate the equilibrium constant at 1123 k for the reaction: c(s) + co2(g) + 2cl2(g) ⇌ 2cocl2(g) 4.68 × 10 9 (enter your answer in scientific notation.) write the equilibrium constant expression, kp:
Answers: 3

Chemistry, 23.06.2019 00:30, natishtaylor1p8dirz
What is the chemical formula of magnesium bromide? a. mgbr2 b. mgbr c. mg2br2 d. mg2br
Answers: 3
Do you know the correct answer?
16. A solution is made by dissolving 25 g of NaCl in enough water to make 1.0 L of solution. Assume...
Questions in other subjects:






Chemistry, 23.04.2020 04:12


Biology, 23.04.2020 04:12


Mathematics, 23.04.2020 04:12