Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, rebeccacruzz2017
Calculate - analysis of compound composed of iron and oxygen yields 174.86 of fe and 75.14g of o. what is the empirical formula for this compound?
Answers: 3

Chemistry, 22.06.2019 12:30, hala201490
Place the elements below in order of decreasing ionization energy. aluminum(al) chlorine(cl) magnesium (mg) sulfur(s)
Answers: 1
Do you know the correct answer?
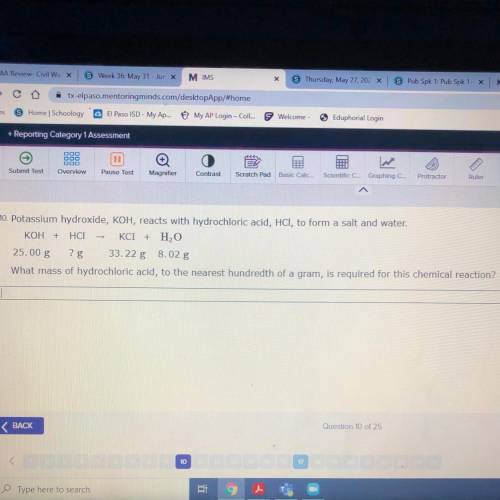
10. Potassium hydroxide, KOH, reacts with hydrochloric acid, HCl, to form a salt and water.
KOH + H...
Questions in other subjects:


History, 25.07.2019 11:50

Biology, 25.07.2019 11:50

Social Studies, 25.07.2019 11:50

History, 25.07.2019 11:50

Social Studies, 25.07.2019 11:50


Business, 25.07.2019 11:50

Social Studies, 25.07.2019 11:50