Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:50, ddmoorehouseov75lc
If a substance is not at its melting or boiling point, as the heat content of a sample of matter increases, its temperature increases the number of intermolecular bonds decreases the space between particles increases the particles move faster
Answers: 2



Chemistry, 22.06.2019 19:30, amandamiro05
Helium decays to form lithium. which equation correctly describes this decay?
Answers: 2
Do you know the correct answer?
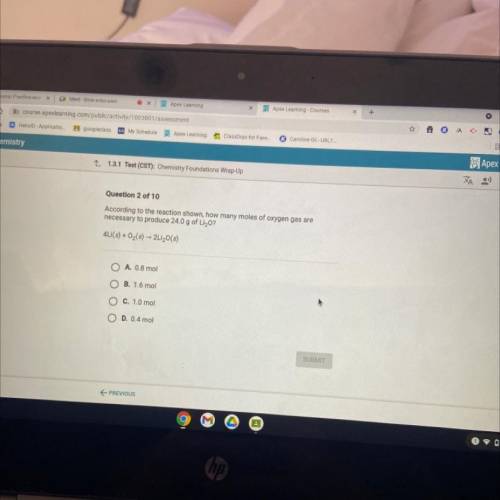
According to the reaction shown, how many moles of oxygen gas are
necessary to produce 24.0 g of Li...
Questions in other subjects:


Mathematics, 29.08.2019 09:50


Biology, 29.08.2019 09:50

Biology, 29.08.2019 09:50

Social Studies, 29.08.2019 09:50

Social Studies, 29.08.2019 09:50



Chemistry, 29.08.2019 09:50