Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:00, uniqueray33
What effect does a decrease in temperature have on the overall rate of a chemical reaction? a decrease in temperature decreases . the reaction rate will
Answers: 1

Chemistry, 22.06.2019 08:30, myamiller558
Which of the following would be an accurate picture of the earth during the summer time of the northern hemisphere?
Answers: 1

Chemistry, 22.06.2019 08:30, MacenParisi
In the reaction between a crushed antacid tablet and vinegar what gas is emitted
Answers: 2

Chemistry, 22.06.2019 09:00, Aminton737
Plz mark brainliest 30 points1) find the momentum of a 12 kg snowball that is rolling with a velocity of 9 m/s.2) an 8 ball with a mass of .5 kg is sitting at rest. it is hit by the cue ball (1 kg) traveling at 2.5 m/s. if the cue ball is at rest after the collision, how fast is the 8 ball traveling after the collision? 3) two football players are running toward each other. if the offensive player is 75 kg and is running 8 m/s, how fast must the 60 kg defensive player run in order for the two players to hit and stop?
Answers: 1
Do you know the correct answer?
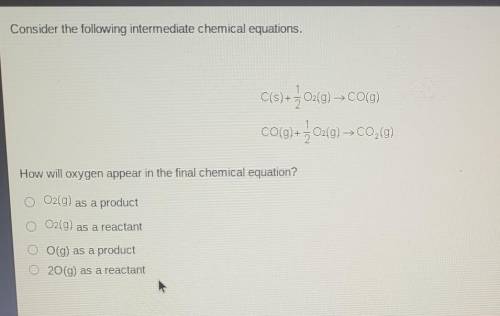
Consider the following intermediate chemical equations.
C(s) + + O2(g) → CO(g) CO(g) + } 02(g) C0,0...
Questions in other subjects:


Social Studies, 12.11.2019 02:31


Geography, 12.11.2019 02:31

Chemistry, 12.11.2019 02:31


History, 12.11.2019 02:31



Mathematics, 12.11.2019 02:31