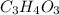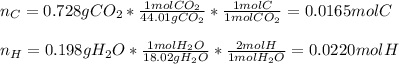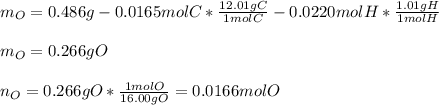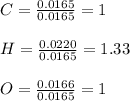Chemistry, 31.05.2021 15:30, bermudezs732
Vitamin C has the formula CxHyOz. You burn 0.486 g of the compound in a combustion analysis chamber and isolate 0.728 g of CO2 and 0.198 g of H2O. What is the empirical formula? Enter the elements in the order C, H, and O.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:30, thickness7699
Pls! plant cells and animal cells were observed under a microscope. the characteristics of two cells are listed below. cell p: does not capture sunlight cell q: has cytoplasm but no chloroplast which statement about the two cells is correct? cell q also has a cell wall. cell q also has large vacuole. cell p also has a large vacuole. cell p also has a cell membrane.
Answers: 1

Chemistry, 22.06.2019 03:10, josephpezza18
The covalent compound acetylene, which is the fuel of the oxyacetylene torch used by welders, has the molecular formula c2h2. the covalent compound benzene, a commercial solvent, has the molecular formula c6h6 each of these covalent compounds contains carbon and hydrogen atoms in a one-to-one ratio. would it be correct to write the chemical formulas of each as ch? explain.
Answers: 1
Do you know the correct answer?
Vitamin C has the formula CxHyOz. You burn 0.486 g of the compound in a combustion analysis chamber...
Questions in other subjects:




Mathematics, 11.12.2019 20:31





Chemistry, 11.12.2019 20:31