

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:50, jordan5778
What is the overall order of reaction for rate = k[no]2[o2]
Answers: 3

Chemistry, 22.06.2019 04:00, amandasantiago2001
Which of the following ocean acidification? are the most likely side effects of a ph less than 7.0 in the ocean b. more metal salts altering the ocean chemistry c. dissolution of the shells of marine organisms d. both a & b e. all of the above.
Answers: 3

Chemistry, 23.06.2019 06:30, OsoDeOro7968
Which of these natural resources is non-renewable a. corn b. wind c. geothermal d. natural gas
Answers: 2
Do you know the correct answer?
Given the following reaction:
2 ZnS(s) + 3 O2(g) → 2 ZnO(s) + 2 SO2(g) ΔH = -927.54 kJ
...
...
Questions in other subjects:

History, 27.11.2019 10:31




Mathematics, 27.11.2019 10:31



Mathematics, 27.11.2019 10:31

Mathematics, 27.11.2019 10:31


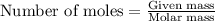 ......(1)
......(1)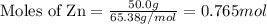
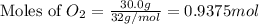

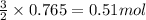 of oxygen gas
of oxygen gas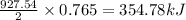 of energy
of energy



