
Chemistry, 30.05.2021 17:00, AtlFan6392
Given the reaction: N2 + O2 = 2NO for which the Keq at 2273 K is 1.2 x 10-4
a. Write the equilibrium constant expression for the reaction.
b. Write the equation that would allow you solve for the concentration of NO.
c. What is the concentration of NO if [NZ] = 0.166M and [02] = 0.145M?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, fastpitchhailey1354
An electrons position cannot be known precisely only it's probability of being in a certain location can be known
Answers: 1


Chemistry, 22.06.2019 12:00, zamariahyou
A5.000 g sample of niso4 h2o decomposed to give 2.755 g of anhydrous niso4. what is the formula of the hydrate? what is the full chemical name for the hydrate? what is the molar mass of the hydrate? niso4•_h2o what is the mass % of water in the hydrate?
Answers: 1
Do you know the correct answer?
Given the reaction: N2 + O2 = 2NO for which the Keq at 2273 K is 1.2 x 10-4
a. Write the equilibriu...
Questions in other subjects:

Physics, 17.09.2020 09:01

Mathematics, 17.09.2020 09:01

Mathematics, 17.09.2020 09:01

Mathematics, 17.09.2020 09:01

History, 17.09.2020 09:01

Mathematics, 17.09.2020 09:01

Mathematics, 17.09.2020 09:01

Mathematics, 17.09.2020 09:01

Mathematics, 17.09.2020 09:01

Mathematics, 17.09.2020 09:01


![K_{eq}=\frac{[NO]^2}{[N_2][O_2]}](/tpl/images/1355/6643/3d926.png)
![[NO]=\sqrt{K_{eq}\times [N_2]\times [O_2]}](/tpl/images/1355/6643/c62a1.png)
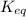
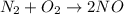
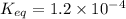
![[N_2]_{eq}=0.166M](/tpl/images/1355/6643/d7b28.png)
![[O_2]_{eq}=0.145M](/tpl/images/1355/6643/728fd.png)
![[NO]=\sqrt{(1.2\times 10^{-4})\times 0.166\times 0.145}](/tpl/images/1355/6643/e15e8.png)
![[NO]=\sqrt{2.88\times 10^{-6}}](/tpl/images/1355/6643/b8658.png)
![[NO]=0.0017 M](/tpl/images/1355/6643/d220d.png)




