
Chemistry, 29.05.2021 22:00, brianna8739
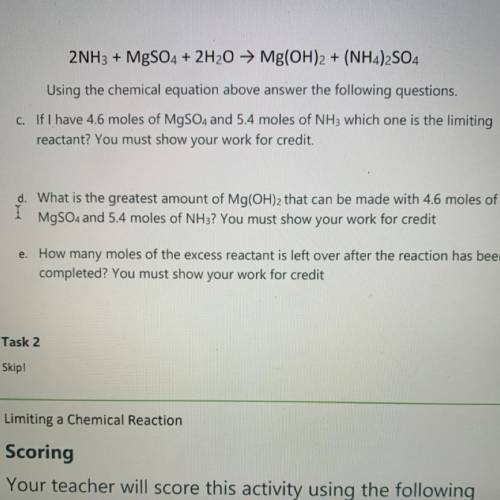
2NH3 + MgSO4 + 2H2O → Mg(OH)2 + (NH4)2SO4
Using the chemical equation above answer the following questions.
C. If I have 4.6 moles of MgSO4 and 5.4 moles of NH3 which one is the limiting
reactant? You must show your work for credit.
d. What is the greatest amount of Mg(OH)2 that can be made with 4.6 moles of
MgSO4 and 5.4 moles of NH3? You must show your work for credit
e. How many moles of the excess reactant is left over after the reaction has been
completed? You must show your work for credit

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:30, dlshadowmorfe
Which of these best describes the scientific process
Answers: 3


Chemistry, 22.06.2019 16:00, winnie45
He table below gives the atomic mass and relative abundance values for the three isotopes of element m. relative abundance (%) atomic mass (amu) 78.99 23.9850 10.00 24.9858 11.01 25.9826 what is the average atomic mass (in amu) of element m? 2.86 5.36 24.30 24.98
Answers: 2

Chemistry, 23.06.2019 05:00, pmbeachy3102
If 15 drops of ethanol from a medicine dropper weigh 0.60g, how many drops does it takes from a dropper to dispense 1.0ml of ethanol? density of ethanol is ethanol is 0.80g/ml.
Answers: 2
Do you know the correct answer?
2NH3 + MgSO4 + 2H2O → Mg(OH)2 + (NH4)2SO4
Using the chemical equation above answer the following qu...
Questions in other subjects:

Mathematics, 22.07.2019 06:30

Mathematics, 22.07.2019 06:30



Business, 22.07.2019 06:30

Biology, 22.07.2019 06:30









