

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:50, deanlmartin
Choose all that apply. when creating a graph, you should: determine the x- and y- variables label the scale on the x- and y- axes plot the data points draw a line of best fit to represent the data trend
Answers: 1


Chemistry, 22.06.2019 01:30, Slycooper5959
Idon't really understand this can you me and show your work.☺☺[ chemistry b] subject [ electron transfer in lonic bonds]grade( 12)
Answers: 1

Chemistry, 22.06.2019 04:00, tifftifftiff5069
You encounter a solution that is acidic and you decide to test it by adding a small amount of a strong acid. the ph lowers slightly but is approximately unchanged, and still remains acidic. what can you say about the solution? a. it is a buffer solution. b. it is not a buffer solution it is a strong acid solution. d. the solution has been neutralized. e. the solution has excess acid present
Answers: 1
Do you know the correct answer?
1. For the reaction 3A — C, the initial concentration of A was 0.2 M, and the reaction rate was
1.0...
Questions in other subjects:

Mathematics, 28.11.2019 14:31





Mathematics, 28.11.2019 14:31


Physics, 28.11.2019 14:31

Social Studies, 28.11.2019 14:31

Mathematics, 28.11.2019 14:31


![r=25M^{-1}s^{-1}[A]^2](/tpl/images/1355/1210/ca3b3.png)
![r=k[A]^n\\\\\frac{r_1}{r_2} =\frac{k[A]_1^n}{k[A]_2^n}](/tpl/images/1355/1210/fb925.png)
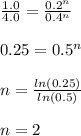
![k=\frac{r}{[A]^n} =\frac{1.0M/s}{(0.2M)^2}\\\\k=25M^{-1}s^{-1}](/tpl/images/1355/1210/0a089.png)




