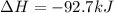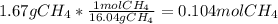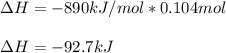Chemistry, 29.05.2021 17:00, allenpaietonp9v8sv
When 1 mol of methane is burned at constant pressure, −890 kJ/mol of energy is released as heat. If a 1.67 g sample of methane is burned at constant pressure, what will be the value of ∆H

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, SmolBeanPotato
What is the volume of the fluid in the graduated cylinder with accuracy and measured to the correct degree of precision? 41.2 ml 42.0 ml 41.23 ml 41.89 ml
Answers: 1

Chemistry, 22.06.2019 11:40, jerrysandoval22
Which of these expressions are correct variations of the combined gas law? p1v1t2 = p2v2t1 both
Answers: 2

Chemistry, 22.06.2019 12:30, azzyla2003
Write the chemical formula for a compound that is made of an element from group 1 and an element from group 17
Answers: 1

Chemistry, 22.06.2019 16:50, mathiscool7
Ajet plane is speeding down the runway during takeoff. air resistance is not negligible. identify the forces on the jet.
Answers: 3
Do you know the correct answer?
When 1 mol of methane is burned at constant pressure, −890 kJ/mol of energy is released as heat. If...
Questions in other subjects:


Law, 10.09.2019 23:30

Mathematics, 10.09.2019 23:30


Mathematics, 10.09.2019 23:30


Mathematics, 10.09.2019 23:30



Spanish, 10.09.2019 23:30