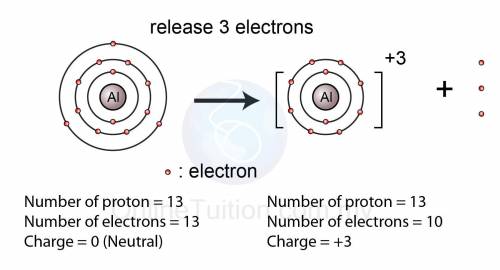
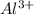An atom has atomic number 13
It looses three electrons to become stable
Name the type of ion...


Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 19:30, simihehe
Phosphorous can form an ion called phosphide, which has the formula p3−. this ion can form an ion called phosphide, which has the formula p3−. this ion properties very similar to those of pforms when a phosphorus atom loses three protonsis called a cationcontains 18 electrons
Answers: 2

Chemistry, 23.06.2019 03:00, andrejr0330jr
Which of the following is a chemical property of water at 4 c
Answers: 2
Do you know the correct answer?
Questions in other subjects:

Mathematics, 05.05.2020 18:13

Computers and Technology, 05.05.2020 18:13



Mathematics, 05.05.2020 18:13

Mathematics, 05.05.2020 18:13