
Chemistry, 28.05.2021 19:50, queenkimm26
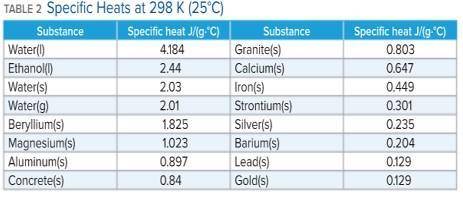
A 136 g sample of an unknown substance was heated from 20.0 °C to 40.0 °C. In the process the substance absorbed 2440 J of energy. What is the specific heat of the substance? Identify the substance among those listed in Table 2.
-A. the specific heat is 0.897 J/g. C, the substance is aluminum
-B. the specific heat is -0.897 J/g. C, the substance is aluminum
-C. the specific heat is 4.184 J/g. C, the substance is water
-D. there's not enough information to determine which is the substance

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:10, tishfaco5000
Answer from each drop-down menu. e characteristics of a borane molecule (bh). the lewis structure and table of electronegativities are given olecular shape is and the molecule is reset next erved. search e a
Answers: 2

Chemistry, 22.06.2019 10:10, babyphoraaaaa
For the reaction, 4 a(g) + 3 b(g) => 2 c(g), the following data were obtained at constant temperature. experiment initial[a],mol/l initial [b],mol/l initial rate, m/min 1 0.200 0.150 5.00 2 0.400 0.150 10.0 3 0.200 0.300 10.0 4 0.400 0.300 20.0 which of the following is the correct rate law for the reaction? 1. rate = k[a]2[b]2 2. rate = k[a][b] 3. rate = k[a]2[b] 4. rate = k[a][b]2
Answers: 3


Do you know the correct answer?
A 136 g sample of an unknown substance was heated from 20.0 °C to 40.0 °C. In the process the substa...
Questions in other subjects:


History, 04.02.2021 21:50

Mathematics, 04.02.2021 21:50



Mathematics, 04.02.2021 21:50


Spanish, 04.02.2021 21:50

English, 04.02.2021 21:50

English, 04.02.2021 21:50






