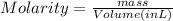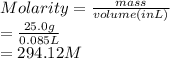Chemistry, 28.05.2021 14:50, brownw2005
What is the molarity when 25.0 g of the compound NaClO3 is placed in 85.0 mL of solution?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:20, UsedForSchool2018
Which of these statements explains the difference between nuclear binding energy and the strong nuclear force ?
Answers: 3

Chemistry, 22.06.2019 23:00, lulprettyb
What is the most common reason for matter changing its state?
Answers: 1

Chemistry, 22.06.2019 23:10, RealStephani
Match the formula for the following compound: magnesium sulfate heptahydratemgs·7h2omg2so4·7h2omg(s o4)2·7h2omgso4·7h2o
Answers: 1

Chemistry, 23.06.2019 04:10, angelina6836
Which of the following is described by the equation h2o(s)+ heat=h2o(i) a freezing melting condensing evaporating
Answers: 2
Do you know the correct answer?
What is the molarity when 25.0 g of the compound NaClO3 is placed in 85.0 mL of solution?...
Questions in other subjects:

Spanish, 16.10.2020 04:01




Chemistry, 16.10.2020 04:01


Mathematics, 16.10.2020 04:01




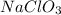 is placed in 85.0 mL of solution is 294.12 M.
is placed in 85.0 mL of solution is 294.12 M.