
Chemistry, 27.05.2021 18:50, reggie36151
WILL GIVE BRAINLIEST TO FIRST QUICKLY PLS
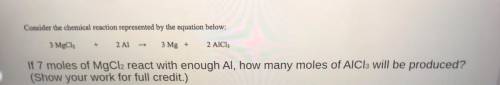
Consider the chemical reaction represented by the equation below:
3 MgCl2 + 2 Al -> 3 Mg + 2AICI
If 7 moles of MgCl2 react with enough Al, how many moles of AlCl3 will be produced?
(Show your work for full credit.)

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, girly37
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 3

Chemistry, 22.06.2019 09:00, dante766
Achemist 16 drop copper metal from copper chloride solution. the chemist place is 0.50 g of aluminum foil in a solution containing 0.75 g of copper (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction?
Answers: 1

Chemistry, 22.06.2019 13:30, bryce99
In a ni-cd battery, a fully charged cell is composed of nickelic hydroxide. nickel is an element that has multiple oxidation states. assume the following proportions of the states: nickel charge proportions found 0 0.17 +2 0.3 +3 0.33 +4 0.5 (a) determine the mean of the nickel charge. enter the answer to 2 decimal places.(b) determine the cumulative distribution function of nickel charge.
Answers: 2

Do you know the correct answer?
WILL GIVE BRAINLIEST TO FIRST QUICKLY PLS
Consider the chemical reaction represented by the equatio...
Questions in other subjects:

Mathematics, 20.11.2020 04:10


Computers and Technology, 20.11.2020 04:10



Mathematics, 20.11.2020 04:10









