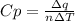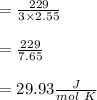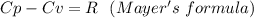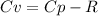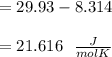Chemistry, 27.05.2021 05:10, genyjoannerubiera
When 229 J of energy is supplied as heat at constant pressure to 3.0mol Ar(g) the temperature of the sample increases by 2.55K. Calculate the molar heat capacities at constant volume and constant pressure of the gas.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:00, hdjsjfjruejchhehd
Define dew point. i am writing this part to be able to ask the question
Answers: 1

Chemistry, 22.06.2019 15:00, makaylajones74pdxtrk
What is the most important factor in determining climates.
Answers: 1

Chemistry, 22.06.2019 18:20, juansebas35
Which reason best explains why metals are malleable? a)because they have delocalized electrons b)because they have localized electrons c)because they have ionic bonds d)because they have rigid bonds
Answers: 2

Chemistry, 23.06.2019 01:00, jazzy200232
Which process results in the release of energy stored in the products of photosynthesis? a. polymer synthesis b. depolymerization c. digestion d. cellular respiration
Answers: 1
Do you know the correct answer?
When 229 J of energy is supplied as heat at constant pressure to 3.0mol Ar(g) the temperature of the...
Questions in other subjects:



Mathematics, 16.12.2019 01:31





Mathematics, 16.12.2019 01:31

History, 16.12.2019 01:31


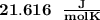 ".
".