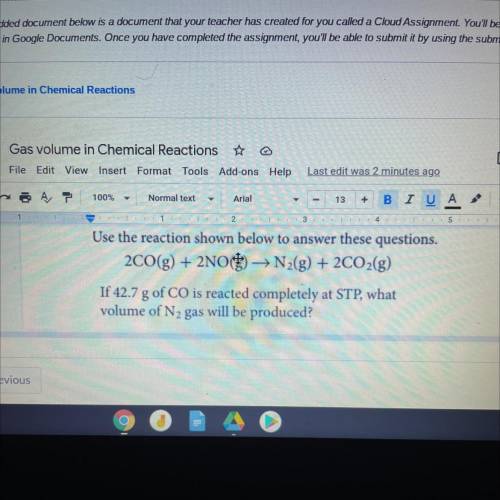
Use the reaction shown l to answer these questions.
2CO(g) + 2NO) → N2(g) + 2CO2(g)
If 42.7 g...

Chemistry, 26.05.2021 19:00, berlyntyler
Use the reaction shown l to answer these questions.
2CO(g) + 2NO) → N2(g) + 2CO2(g)
If 42.7 g of CO is reacted completely at STP, what
volume of N2 gas will be produced?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:50, toniawu18
Problem page gaseous ethane reacts with gaseous oxygen gas to produce gaseous carbon dioxide and gaseous water . if of water is produced from the reaction of of ethane and of oxygen gas, calculate the percent yield of water. be sure your answer has the correct number of significant digits in it.
Answers: 2

Chemistry, 22.06.2019 10:00, shayneseaton
The tendency of water molecules to stick together is referred to as a) adhesion b) polarity c) cohesion d) transpiration e) evaporation
Answers: 1


Chemistry, 22.06.2019 17:30, nijanicole164
A650 ml sodium bromine solution has a bromide ion concentration of 0.245 m. what is the mass (g) of sodium bromide in solution? a) 103.b)0.00155.c)16400.d) 16.4.e) 0.159
Answers: 2
Do you know the correct answer?
Questions in other subjects:

Social Studies, 12.12.2020 16:10

Mathematics, 12.12.2020 16:10



Mathematics, 12.12.2020 16:10



Chemistry, 12.12.2020 16:10

Mathematics, 12.12.2020 16:10







