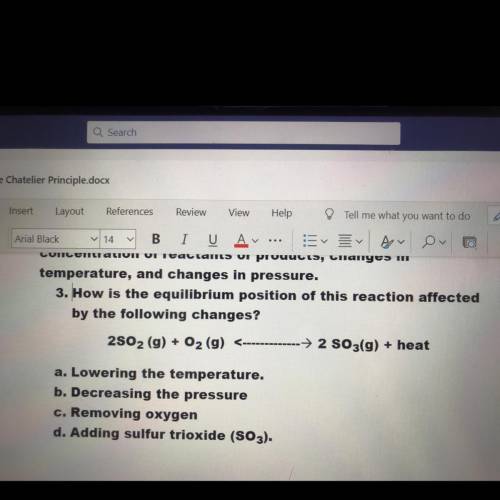
3. How is the equilibrium position of this reaction affected
by the following changes?
2802 (...

Chemistry, 26.05.2021 16:50, hhomeschool24
3. How is the equilibrium position of this reaction affected
by the following changes?
2802 (g) + O2 (g) <-→ 2 S03(g) + heat
a. Lowering the temperature.
b. Decreasing the pressure
c. Removing oxygen
d. Adding sulfur trioxide (SO3).

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:50, Makoshark6887
According to coulomb's law, how would the electrical force between particles change if the product of their electrical charge increased?
Answers: 1


Chemistry, 23.06.2019 06:00, lanaiheart7
What are the coefficients to balance the following equation? ba+br2=babr2
Answers: 2
Do you know the correct answer?
Questions in other subjects:

Mathematics, 16.08.2021 19:20



Business, 16.08.2021 19:20

English, 16.08.2021 19:20

Mathematics, 16.08.2021 19:20


Arts, 16.08.2021 19:20








