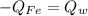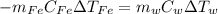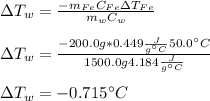Chemistry, 26.05.2021 07:30, jadajordan1010
Please help!
When a 200.0 g block of iron is placed into 1500.0 g of water, the temperature of the iron increases by 50.0 °C. The specific heat of iron is 0.449 J/g^ C . What is the temperature change of the water? (0.715 C)
Thank you!

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, tifftifftiff5069
You encounter a solution that is acidic and you decide to test it by adding a small amount of a strong acid. the ph lowers slightly but is approximately unchanged, and still remains acidic. what can you say about the solution? a. it is a buffer solution. b. it is not a buffer solution it is a strong acid solution. d. the solution has been neutralized. e. the solution has excess acid present
Answers: 1

Chemistry, 22.06.2019 17:00, destinyycooper
What is the approximate vapor pressure when the gas condenses at 70 degrees celsius
Answers: 2

Chemistry, 23.06.2019 00:20, HernanJe6
Steam reforming of methane ( ch4) produces "synthesis gas," a mixture of carbon monoxide gas and hydrogen gas, which is the starting point for many important industrial chemical syntheses. an industrial chemist studying this reaction fills a 1.5 l flask with 3.5 atm of methane gas and 1.3 atm of water vapor at 43.0°c. he then raises the temperature, and when the mixture has come to equilibrium measures the partial pressure of carbon monoxide gas to be 1 .0 atm. calculate the pressure equilibrium constant for the steam reforming of methane at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 1
Do you know the correct answer?
Please help!
When a 200.0 g block of iron is placed into 1500.0 g of water, the temperature of the...
Questions in other subjects:



History, 12.12.2020 16:00




History, 12.12.2020 16:00

Mathematics, 12.12.2020 16:00


English, 12.12.2020 16:00