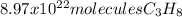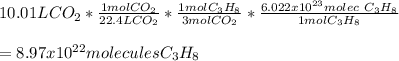Chemistry, 26.05.2021 06:20, darkremnant14
Propane is used as a fuel for camp stoves. It undergoes combustion to form carbon dioxide and water.
C3H3 +502 – 3 CO2 + 4H20
Determine the number of molecules of propane needed to produce 10.01 liters of carbon dioxide

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:20, valencial0917
Why is an elements atomic mass not listed as a whole number on the periodic table
Answers: 2


Chemistry, 22.06.2019 23:00, Mw3spartan17
What extra step distinguishes fermentation from glycolysis
Answers: 1

Chemistry, 23.06.2019 07:20, msladycie8831
F1.5 mol of nabh4 react, how many moles of b2h6 are formed? 2 nabh4(aq) + h2so4(aq) → 2 h2(g) + na2so4(aq) + b2h6(g)
Answers: 1
Do you know the correct answer?
Propane is used as a fuel for camp stoves. It undergoes combustion to form carbon dioxide and water....
Questions in other subjects:





Mathematics, 12.10.2019 15:00

Computers and Technology, 12.10.2019 15:00

History, 12.10.2019 15:00


History, 12.10.2019 15:00

Advanced Placement (AP), 12.10.2019 15:00